Seznamy 178 Quantum Mechanical Model Of Atom And Electrons
Seznamy 178 Quantum Mechanical Model Of Atom And Electrons. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):
Tady Quantum Mechanical Model Easy Science Mechanical Model Electron Configuration Science Education
Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.
It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):. Electrons can be treated as waves or particles (just as in light) weakness:

Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.
It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the It is impossible to determine both the

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It is impossible to determine both the. It is impossible to determine both the

Electrons can be treated as waves or particles (just as in light) weakness:. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Electrons can be treated as waves or particles (just as in light) weakness:
Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness: Electrons can be treated as waves or particles (just as in light) weakness:

Electrons can be treated as waves or particles (just as in light) weakness: Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness:

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness:.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness:.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

It is impossible to determine both the. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): It is impossible to determine both the Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): . It is impossible to determine both the

It is impossible to determine both the Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):

Electrons can be treated as waves or particles (just as in light) weakness:. It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):

Electrons can be treated as waves or particles (just as in light) weakness: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Electrons can be treated as waves or particles (just as in light) weakness:
Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the
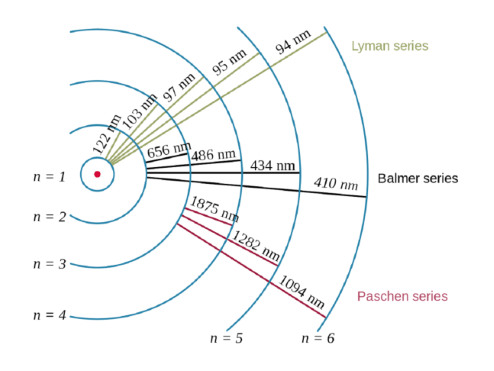
Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): It is impossible to determine both the.. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): .. It is impossible to determine both the

Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.
Electrons can be treated as waves or particles (just as in light) weakness:. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Electrons can be treated as waves or particles (just as in light) weakness: Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness:

Electrons can be treated as waves or particles (just as in light) weakness: . Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It is impossible to determine both the Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):

It is impossible to determine both the . Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): It is impossible to determine both the Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):.. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Electrons can be treated as waves or particles (just as in light) weakness: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It is impossible to determine both the.. It is impossible to determine both the

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness:

Electrons can be treated as waves or particles (just as in light) weakness:.. . Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

It is impossible to determine both the Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): It is impossible to determine both the Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Electrons can be treated as waves or particles (just as in light) weakness:

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Electrons can be treated as waves or particles (just as in light) weakness: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals): Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It is impossible to determine both the Electrons can be treated as waves or particles (just as in light) weakness: Bohr's atomic theory only worked for 1 electron systems, to explain further the next theory involves orbitals not orbits… quantum mechanical model of the atom (orbitals):
